Free Control Substance Inventory Michigan Template in PDF
The Control Substance Inventory Michigan form is an essential document for registered locations handling controlled substances within the state. This annual inventory must be conducted between April 1 and June 30 each year, ensuring that all controlled substances are accounted for accurately. Each registered location is required to complete a separate inventory, reflecting the specific substances held at that site. Once completed, the form should be mailed to the State of Michigan's Bureau of Health Professions, and a signed copy must be retained at the licensed location for record-keeping purposes. This document not only fulfills state requirements but can also serve as the biennial inventory mandated by the Drug Enforcement Administration (DEA). Key details captured on the form include the date of the inventory, the name and address of the DEA registrant, and specific information about the controlled substances, such as their schedule, container type, quantity, and concentration. Notably, Schedule I and II substances must be documented separately to maintain compliance with regulatory standards. The form also requires signatures from the individual performing the inventory and a witness, ensuring accountability throughout the process.
Form Example
Michigan State University
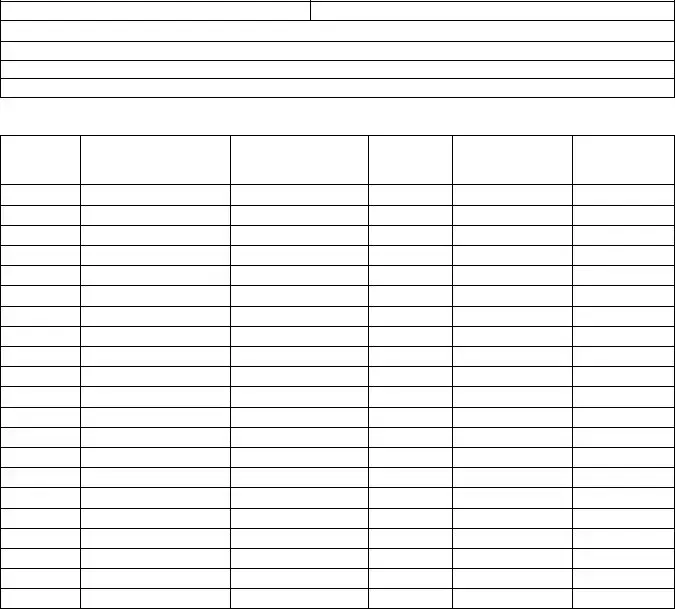
Annual Controlled Substance Inventory Form
Inventory must be performed between April 1 and June 30 of each year. A separate annual inventory is required for each registered location. Mail to: State of Michigan, Bureau of Health Professions‐ Health and Regulatory Division, Annual Inventory, 6546 Mercantile Way, Suite 2, P.O. Box 30454, Lansing, MI 48909. Retain a signed and completed copy of this form at the licensed location. The completed form can serve as the biennial inventory required by the DEA.
Date:
Start of day |
End of day |
MI Licensee/DEA Registrant Name:
MI Licensee/DEA Registrant Address:
DEA Registration #:
State of MI Controlled Substance ID #:
DEA Schedule*
Controlled Substance
Container Unit Type (Vial, syringe, patch, etc.)
Container Quantity
Container Volume
Concentration
*Schedule I and II controlled substances must be separated from all other substances or places on a separate form.
Inventory performed by: _________________________________ |
____________________________________________ |
Print Name |
Signature |
Inventory witnessed by: _________________________________ |
____________________________________________ |
Print Name |
Signature |
|
Page: ___ of_ __ |
Document Specs
| Fact Name | Description |
|---|---|
| Inventory Period | The inventory must be conducted annually between April 1 and June 30. |
| Location Requirement | A separate inventory is mandatory for each registered location. |
| Mailing Address | Completed forms should be mailed to the Bureau of Health Professions at 6546 Mercantile Way, Suite 2, P.O. Box 30454, Lansing, MI 48909. |
| Retention Requirement | A signed and completed copy of the inventory form must be retained at the licensed location. |
| DEA Compliance | The completed inventory form can fulfill the biennial inventory requirement set by the DEA. |
| Data Entry | Information required includes the date, licensee/registrant name, address, DEA registration number, and controlled substance details. |
| Separation of Schedules | Schedule I and II controlled substances must be listed separately or documented on a different form. |
| Witness Requirement | Both the person performing the inventory and a witness must sign the form. |
Fill out Common Templates
Mc 230 - It includes safeguards for the management and release of particularly sensitive health information like psychological records.
For those looking to navigate the legalities of mobile home ownership, our informative guide on the Mobile Home Bill of Sale requirements provides invaluable insights into the necessary steps and documentation needed for a successful transaction.
Michigan Scao Forms - Permits the legal system to efficiently manage and close cases involving financial judgments.